Learn about equipment validation in pharmaceutical industry and how to perform it.
Equipment Validation in pharmaceutical industry is defined as “documented evidence, which provides a high degree of assurance that a specific process will constantly produce a product meeting its pre-determined specifications and quality attributes.” In other words, equipment validation provides documented evidence that a process or equipment is capable of producing the required results and having the required quality
Equipment validation in pharmaceutical industry is an integral process in the pharmaceutical industry that validates the ongoing processes, equipment, and personnel against quality variables. It also helps to detect deviations that could occur during routine operations. Different regulatory bodies, such as FDA and the WHO, inspect and review validation activity as a mandatory requirement. They can issue a warning or non-conformance if validation activity is not performed appropriately.
The validation is required every time a new process initiates. After the first time, it is performed at regulator intervals to ensure consistency in producing the desired results.
Equipment validation in pharmaceutical industry
Equipment validation in pharmaceutical industry tests various systems and processes against standard acceptance criteria. If equipment fulfills the acceptance criteria, validation becomes satisfactory, and equipment is allowed for production.

On the other hand, if the validation result does not meet the acceptance criteria, the maintenance department must remove the fault or deficiency before continuing with production. After rectification of fault, equipment is again validated, and only if it passes the acceptance criteria, is production allowed for that particular equipment.
Who performs the equipment validation in pharmaceutical industry?
The Quality Department is responsible for carrying out equipment validation in pharmaceutical industry. Other departments are responsible for supporting the Quality department in the validation process.
Before executing the validation activity, a team comprising members from all concerned departments is formed. This team must always validate equipment according to the regulatory requirements and company policies.
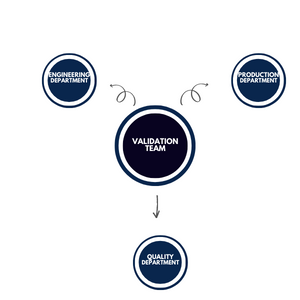
Commonly, members from the following departments are included in the team
Quality Department
The quality department is responsible for executing the Validation activity. It is granted rights to use the entire organization’s resources. Additionally, the quality department is the decision-maker throughout the validation activity.
Engineering Department:
The engineering department is responsible for providing engineering support during the validation activity. They are also responsible for analyzing the machine’s performance, and taking corrective or preventive actions if required.
Production Department
Validation must include a representative of the Production department where the machine is installed or is under production. For example, the injectable department’s supervisor or representative must be included in the team for validating the ampoule filling machine. Because production personnel is the custodian of pharma equipment. They must be involved in the qualification process of said equipment.

Process of equipment validation in pharmaceutical industry
During validation, equipment is validated in four stages, as mentioned below.
- Design Qualification
- Installation Qualification
- Operational Qualification
- Performance Qualification
Let’s look at what each means and how to perform
Design Qualification – DQ
Design Qualification-DQ is the approval phase of equipment’s design specification. It is documented evidence that the equipment specification meets the buyer’s requirements. The proposed equipment will produce products that meet quality, safety, and regulatory attributes.
DQ is prepared by the equipment manufacturer and is sent to the equipment buyer for approval. The buyer company verifies the DQ and proposes changes if required. After the buyer and manufacturer agree on DQ, both parties sign it, and the copy is retained.
Installation Qualification – IQ
Installation Qualification: IQ is written evidence that the pharma equipment is installed according to the manufacturer’s specifications and recommendations. The equipment has all the recommended utilities, such as Compressed Air, Steam, and Electrical Power.
Other checklists include
Documentation
Before installation, it is verified that the manufacturer has supplied all the documentation, such as the operating Manual, Maintenance Manual, and Wiring Diagram. These documents are essential because they are critical for safe machine operation. They help the maintenance and production personnel during machine problems or troubleshooting.
Material of Construction
Pharmaceutical equipment must be constructed from a material that does not harm the pharma products. The equipment part that comes in direct contact with the product must be constructed with SS 316L. The part not contacting the pharma product must be constructed with SS 304.
Additionally, it must meet the surface finish requirements for pharmaceutical equipment.
Calibration certificates
The manufacturer must provide certificates for all measuring and recording devices used with the machine. The certificates must be valid and must not be expired.
Accessories Installation
Verification that all the attached ancillary instruments and components, such as temperature sensor and pressure transmitter, are present and properly installed.
Operational Qualification – OQ
Operational Qualification – OQ is written evidence that the installed pharma equipment operates and functions according to the user or buyer’s requirement. It ensures that its operation is according to the specification agreed upon during the design phase of the discussion.
In this test, equipment is operated, and its functions are verified individually. If any discrepancy is found, it is noted and immediately informed to the manufacturer.
Some functions tested during the Operational Qualification test include the following.
Basic Machine Operation
it includes running the machine and checking to see the normal machine behavior and whether there are any abnormalities.
Safety System
it includes verifying equipment’s safety systems such as Emergency Button, Operator Safety system, Electrical Safety, and Mechanical safety system.
Capacity
The capacity of the equipment is verified in terms of speed and production output.
Motion Control
If motion control systems are involved in machine operation, they are verified for satisfactory working.
Temperature Control System
Equipment with temperature control functions is tested before the machine is operated.
Instrumentation Check
Instruments such as pressure and humidity monitoring devices are checked for the correct functioning.
Performance Qualification – PQ
Performance Qualification – PQ is the final stage of equipment validation in pharmaceutical industry. After satisfactory PQ results, the machine is allowed for the production process.
In PQ, machine operation is tested as a whole rather than testing individual sections or functions. The machine is operated with actual machine parameters and actual products. Its operation is verified against the specification agreed upon during the design phase.
Additionally, the quality department performs laboratory tests on the pharma product to verify its effectiveness.

